HOW DOES CORROSION INHIBITOR WORK
Introduction
Corrosion has posed a lot of problems to various companies and industries; by interfering with the finances and profits. The discovery of corrosion inhibitors solves the problem of spending huge amounts annually on combating Corrosion by industries.
At GZ Industrial supplies, we deal on chemicals such as corrosion inhibitors that help prevent the onset of Corrosion on your equipment, machines, tools as well as your automobiles using our different types of corrosion inhibitors. One of the challenges industries and companies face is keeping up with these developments, such as difficulties in keeping equipment and machines functional in various stages of Corrosion; read on to find out how corrosion inhibitors work.
Read more...Why Is It Important To Eliminate Rust Or Corrosion From Your Businesses Facilities?
Key Takeaway:
- Corrosion inhibitors are chemical compounds that slow down or prevent the corrosion of metal surfaces.
- They work by forming a protective film or barrier over the metal, isolating it from corrosive agents like water, oxygen, and salts.
- These inhibitors can be applied through coatings, added to fluids, or introduced directly into systems like pipelines or cooling towers.
- There are different types, including anodic, cathodic, mixed, and vapor-phase inhibitors, each suited to specific environments and metals.
What is a Corrosion Inhibitor?
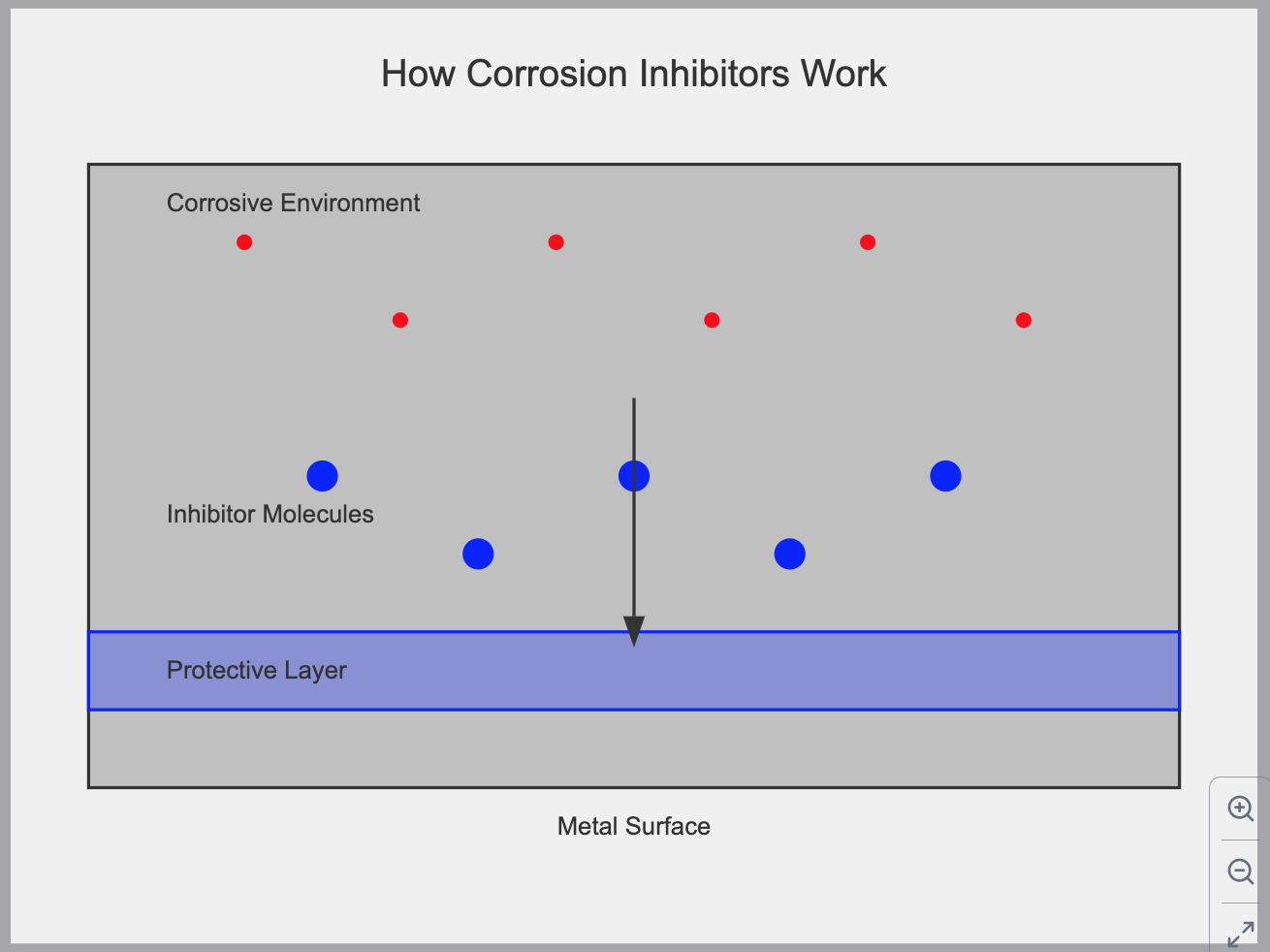
This is a chemical substance that is applied to a particular material, mainly metals, and also in the environment to drastically reduce the rate of Corrosion of that material. This is made possible by a coating process on the surface of the metal acting as passivation restricting access of water, air, and dirt, etc., directing on the metal surface. This is seen as the first line of defense against Corrosion.
Corrosion Inhibitors are basically applied;
- On surfaces of metals
- In coolants, fuels, hydraulic fluids, boiler water, engine oil, and many other fluids used in industries and companies.
- In the concrete mixture, when pouring concrete with steel rebar.
Corrosion inhibitors usually consist of some particular amounts of Nitrogen-based compounds or Amines, a diluent or solubilizer, oxyalkylated naphthenic acid, and acetylenic alcohol or sulfide.
Scientific Explanation of Corrosion Inhibitor Mechanisms
To provide a more in-depth understanding of how corrosion inhibitors work, let's explore the scientific principles behind their mechanisms:
Adsorption Mechanism:
- Corrosion inhibitors adsorb onto the metal surface, forming a protective film.
- This process involves either physical adsorption (van der Waals forces) or chemisorption (chemical bonding).
- The adsorbed layer acts as a barrier, preventing corrosive species from reaching the metal surface.
Passivation:
- Some inhibitors promote the formation of a passive oxide layer on the metal surface.
- This layer is typically thin, dense, and adherent, providing excellent corrosion resistance.
- Examples include chromates and nitrites, which form protective oxide films on steel.
Cathodic Inhibition:
- Cathodic inhibitors work by slowing down the reduction reaction at cathodic sites.
- They often form insoluble precipitates on cathodic areas, reducing the available surface for the cathodic reaction.
- Zinc and calcium salts are common cathodic inhibitors.
Anodic Inhibition:
- Anodic inhibitors target the oxidation reaction at anodic sites.
- They typically form a protective oxide film, raising the anodic potential and moving the metal into its passivation region.
- Examples include chromates, molybdates, and nitrites.
Vapor Phase Inhibition:
- Volatile corrosion inhibitors (VCIs) work in the vapor phase.
- They sublimate and condense on metal surfaces, forming a protective molecular layer.
- This is particularly useful for protecting metals in enclosed spaces during storage or shipping.
Understanding these mechanisms allows for more effective selection and application of corrosion inhibitors in various industrial settings.
Read more....Why Is It Important To Eliminate Rust Or Corrosion From Your Businesses Facilities?
The Effects and Solution for Corrosion
A reaction of metals with substances such as water, oxygen, hydrogen, dirt, bacteria, or even an electrical current can result in the Corrosion of metals. Metals like steel corrode when they are put under some stress subjection resulting in cracks, and after various types of research, we at Gz Industrial supplies offer the use of corrosion inhibitors in combating rust.
EFFECTS:
- Corrosion brings about high expenses as a result of replacing new machines and tools.
- The maintenance methods such as painting bring about high costs.
- It reduces the machine and equipment efficiency.
- It causes the damage of products in the corroded metal container.
- It requires safety measures to be carried out to prevent the release of hazardous chemicals.
- Corrosion causes health problems when a product from such a source is accumulated in the body. For example, drinking water from a contaminated lead pipe.
SOLUTIONS:
The application and uses of corrosion Inhibitors are widely seen in Companies, commercial processes, and industrial environments. Their uses include:
- To stop rusting and anodic Corrosion of metals by coating the metal surface with a chromate layer.
- Corrosion inhibitors like the Oxygen scavengers react with dissolved oxygen in the environment and prevent cathodic Corrosion.
- They are also used in securing fuel pipelines, preventing rust, and reducing the risk of accidents.
- Corrosion inhibitors play important roles in also securing Metal pipes prone to Corrosion in heating systems.
Read more...Why Choose Us for Your Corrosion Protection Needs: Our Expertise and Dedication to Quality
Comprehensive Overview of Corrosion Inhibitors
Types of Corrosion inhibitors
Epochem Corrosion inhibitors are classified into two general types due to the nature in which they prevent Corrosion. Some corrosion inhibitors also serve as an industrial coolant and protect engines’ cooling systems, while others reduce the corrosion rate of metals. They include;
Water-based inhibitor
The water-based inhibitor is an Epochem brand of Cleaning and maintenance chemicals in Nigeria. This corrosion inhibitors coolant is a series of extended-life industrial coolant/antifreeze and heat transfer fluids formulated for stationary equipment applications already pre-diluted. These products are based on a unique extended-life carboxylate inhibitor system and nitrite/molybdate as secondary inhibitors. These water-based inhibitors provide complete protection of cooling system components, do not contain borates, phosphate, amines, and silicates, and are available in ethylene glycol-based fluid.
Solvent-based inhibitor
The solvent-based Epochem brand of Cleaning and maintenance chemicals in Nigeria. It is applied to an environment that significantly reduces the corrosion rate of materials, especially metals exposed to that environment. It is considered the first line of defense against Corrosion.
These solvent-based corrosion inhibitors are corrosion preventatives for application to surfaces likely to corrode, either in storage or in use. They are blends of selected rust prevention materials dissolved in a solvent for ease of application. On application, the solvent evaporates, leaving a protective coating.
How Corrosion Inhibitors Work
There are three basic mechanisms at which corrosion inhibitors work regardless of the type of inhibitors being used as well as their environment;
- By the process of chemical absorption, the inhibitor molecule readily diffuses into the metal surface; either by self or with Interaction with the metallic ions, it then forms a thin protective film in the form of a precipitate.
- This protective film formed as a result of the reaction between the inhibitor and metal produces metal oxides which is what increases the corrosion resistance.
- The inhibitor reacts with a potentially corrosive substance in the water.
How to choose the most suitable corrosion inhibitor
- Consider the nature and type of materials to be protected
- The type of corrosion inhibitor to be used(Solvent, oil, or water-based)
- Application method (dip, spray, brush, etc.)
- Protection type required (in process, storage, or shipping)
- Nature of thickness and type of coating residue desired
- Temperature, humidity, storage, packaging, and/or shipping conditions of the material.
- Other Interactions with subsequent processes.
- Requirement for safety and environmental health.
Real-World Applications of Corrosion Inhibitors
To illustrate the practical importance of corrosion inhibitors, here are some real-world applications across various industries:
Oil and Gas Industry:
- Corrosion inhibitors are added to pipelines to protect against internal corrosion caused by water, CO2, and H2S.
- Example: Use of imidazoline-based inhibitors in crude oil pipelines reduces corrosion rates by up to 95%.
Automotive Industry:
- Coolant systems in vehicles use corrosion inhibitors to protect radiators and engine blocks.
- Example: Ethylene glycol-based coolants with silicate and phosphate inhibitors extend engine life by preventing rust formation.
Marine Applications:
- Ships use corrosion inhibitors in ballast tanks and cooling systems to combat the harsh saltwater environment.
- Example: Vapor phase inhibitors are used in void spaces of naval vessels, reducing maintenance costs by up to 50%.
Construction:
- Corrosion inhibitors are added to reinforced concrete to protect steel rebar from corrosion.
- Example: Calcium nitrite inhibitors in bridge decks can extend service life by 20-25 years.
Water Treatment:
- Cooling towers and boilers use corrosion inhibitors to protect metal surfaces from scale and corrosion.
- Example: Phosphonate-based inhibitors in industrial cooling systems reduce corrosion rates by up to 99%.
Aerospace:
- Aircraft use corrosion inhibitors in fuel tanks and hydraulic systems to prevent metal degradation.
- Example: Chromate-based inhibitors in aluminum alloys increase the fatigue life of aircraft structures by up to 40%.
These examples demonstrate the critical role of corrosion inhibitors in protecting assets, improving safety, and reducing maintenance costs across various sectors.
Frequently Asked Questions
1. How long does it take for a corrosion inhibitor to become effective?
The effectiveness of a corrosion inhibitor can vary depending on the type and application method. Some fast-acting inhibitors can provide protection within hours, while others may take 24-48 hours to form a complete protective layer. For optimal results, always follow the manufacturer's recommendations for application and curing time.
2. Are corrosion inhibitors environmentally friendly?
Many modern corrosion inhibitors are designed to be environmentally friendly. Green inhibitors derived from plant extracts, such as tannins and amino acids, are becoming increasingly popular. However, some traditional inhibitors may contain harmful substances. Always check the environmental impact and disposal requirements of the specific inhibitor you're using.
3. Can corrosion inhibitors be used on all types of metals?
While there are corrosion inhibitors available for most metals, not all inhibitors are suitable for all metal types. Some inhibitors are specifically formulated for ferrous metals, while others work best on non-ferrous metals like copper or aluminum. It's crucial to select an inhibitor that's compatible with your specific metal and application.
4. How often should corrosion inhibitors be reapplied?
The reapplication frequency depends on the type of inhibitor, environmental conditions, and the level of protection required. Some long-lasting inhibitors can provide protection for several years, while others may need reapplication every few months. Regular inspections and following manufacturer guidelines are key to maintaining effective corrosion protection.
5. Can corrosion inhibitors be used in combination with other protective methods?
Yes, corrosion inhibitors are often used as part of a comprehensive corrosion protection strategy. They can be combined with protective coatings, cathodic protection systems, or material selection to provide enhanced corrosion resistance. However, it's important to ensure compatibility between different protection methods to avoid adverse interactions.
Related Articles
The Best Rust preventative| Anti-Corrosion fluid in Nigeria
Corrosion Management in the Niger Delta: Innovative Solutions for Oil and Gas Infrastructure
Key Factors to Consider When Implementing Corrosion Protection Measures
Conclusion
In order to protect, prevent and prolong the life of your metallic equipment, tools, and machines, Professionals have resulted the use of corrosion inhibitors as a conservation and restoration method of these materials. Corrosion protection is a necessary requirement for all major equipment and machines.
At GZ Industrial supplies our products and services are marked with the highest level of corrosion management standard that ensures all our clients receive quick and quality service, with our corrosion inhibitor working and giving outstanding protection against Corrosion and rust in a matter of a few hours.






